MEDELIA – the first modular software suite for MDR compliance in Post Market management and eIFU for medical devices
MEDELIA represents the evolution of our original eIFU software module for electronic Instructions For Use digitization. Born from listening to client needs, it has grown into a comprehensive ‘medical suite’ designed to address manufacturing companies’ diverse requirements within ever-changing regulatory landscapes.
We are proud partners of BioPmed, Piedmont Region’s leading innovation hub for Health and Life Sciences.
Explore the MEDELIA suite and its modules!
Download brochure Request a demo
A modular solution software that grows with your company
The modular approach ensures you get a solution tailored to today’s requirements while providing the flexibility to scale and adapt to tomorrow’s new challenges and demands.
Currently, the suite is dedicated to the needs of companies that manufacture medical devices and in vitro diagnostics, but it is constantly expanding.
Discover the advantages of switching to software
A complete suite for MDR compliance
Our vision extends far beyond the simple digitization of isolated processes. We embrace a holistic approach to MDR compliance, enabling different software modules to communicate seamlessly and create powerful synergies. This strategic perspective drives our evolution of Medelia to integrate electronic Instructions For Use (eIFU) management with PMS and PMCF systems.
Cost savings
Up to 70% less time!
Our clients report a significant reduction in time dedicated to post-market data management, with savings that can reach 70% for some critical activities such as PSUR report compilation.
Full traceability
Stop scattered documents!
Medelia automatically records every action performed in the system, creating a detailed audit trail that greatly simplifies regulatory inspections and compliance checks.
Efficiency and Scalability
Medelia is “API first”!
Integration with other corporate systems is simple. We connect with quality management systems, CRM, and ERP platforms, creating a unified digital ecosystem that eliminates redundant data entry and duplicated efforts.
MEDELIA is designed for manufacturing companies, but we aim to support all market stakeholders including regulatory affairs professionals and consulting companies. If you represent one of these organizations, please contact us for additional information.
MDR Updates January 2025
The recent European Commission proposal to extend the scope of eIFU application to all medical devices intended for healthcare professionals makes this integrated approach even more relevant. Companies adopting comprehensive digital solutions like MEDELIA now will find themselves in an advantageous position when these regulatory changes come into effect.
Tackle digital transformation and regulatory compliance adaptation with a single technology partner.
Module Overview & Features
Discover MEDELIA modules
This suite creates a virtuous cycle: data collected through the PMS system identifies the need to update Instructions For Use, the update happens quickly thanks to the eIFU module, and feedback on the new content is collected again by the PMS system, enabling continuous improvement based on real data.
Discover all the features available through our modules: eIFU, PMS and PMCF.
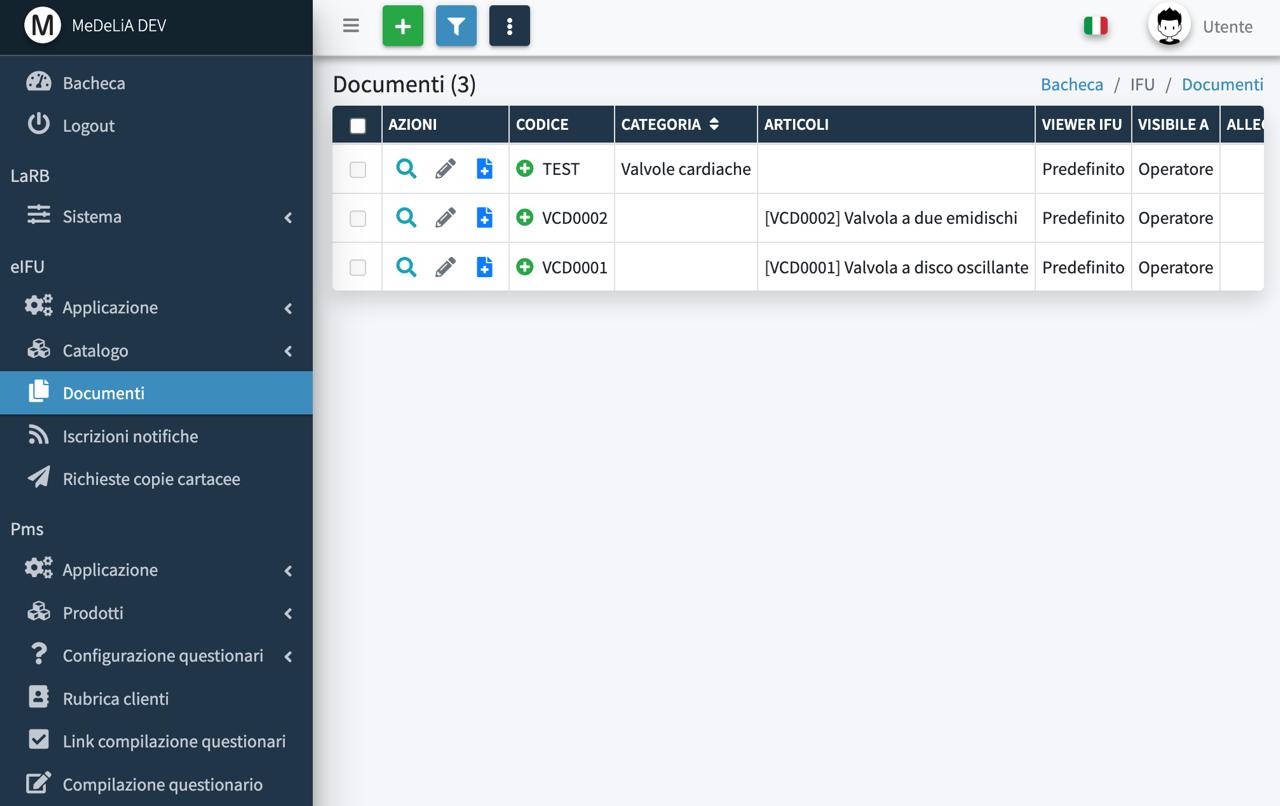
eIFU Module
Instant revision publishing
Instant notifications to healthcare professionals
Multi-language document management, products and lot tracking
QR Code for immediate eIFU access
Simple and advanced search on web portal
Customizable branding and audience targeting on web porta
PMS / PMCF Module
Monitoring dashboard
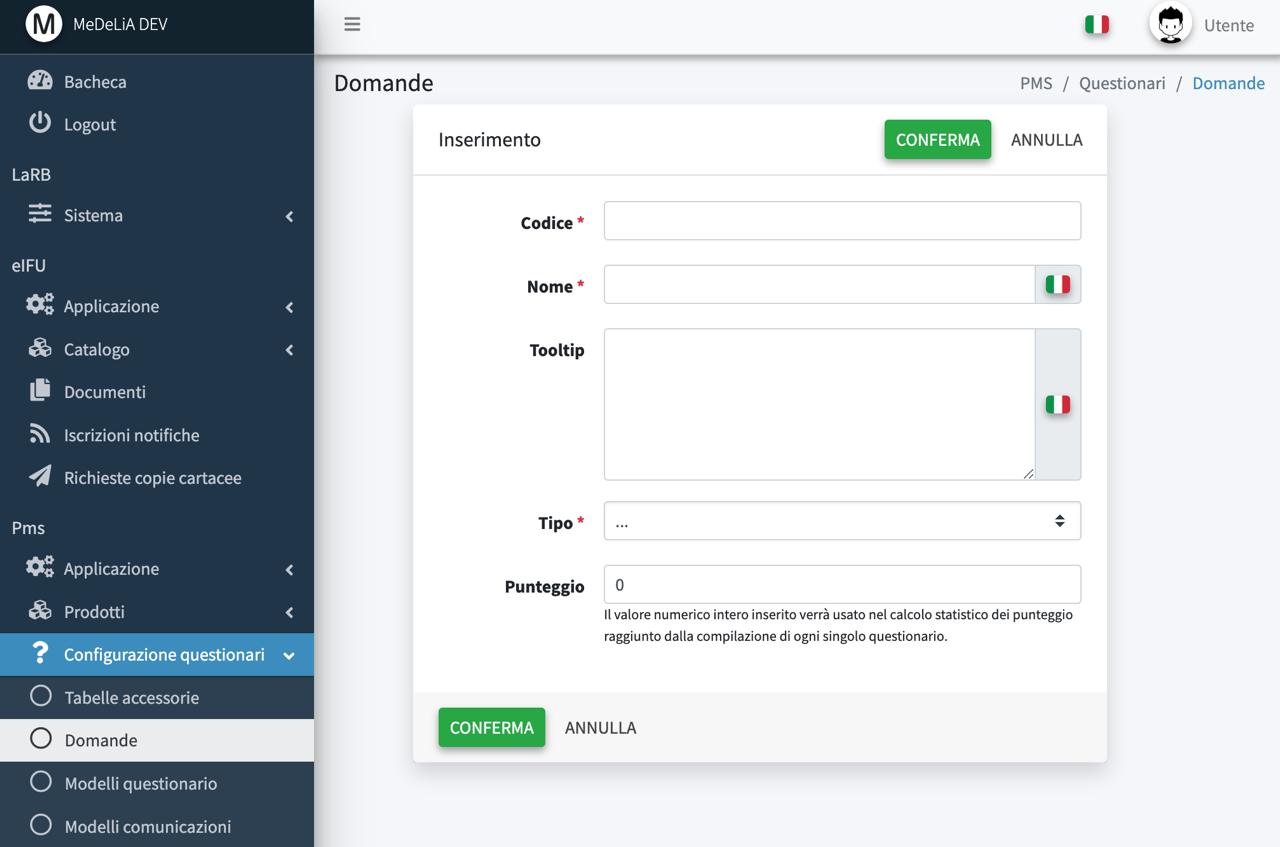
Customized questionnaires and clinical data collection modules
Threshold levels / scoring, notification management, follow-up and alarms (configurable)
Statistics and charts
Flexible setup for both direct and indirect customer relationships
EUDAMED report generation (in development)
WHAT’S NEW IN 2025
We’re developing QRA!
The new module will manage the technical documentation necessary for medical device market placement.
This integration will further streamline regulatory compliance management and represents another step toward MEDELIA’s goal of offering a complete suite dedicated to medical device manufacturers.
Trust a recognized technology partner in the industry!
Cloud & On-premise versions available

Our Cloud solution leverages regulation-compliant, enterprise-grade servers with built-in redundancy and automated backup systems.

Our On-Premise option gives you complete control by running on your own infrastructure. Check our brochure for technical requirements.
Blog
Read our latest articles
medelia is certification ready
A regulatory landscape in constant evolution
Every MEDELIA module complies with European regulations. RealBit provides each software module with the validation documents required by current standards (FDA and MDR), ensuring that the software is ready to pass your certification process.
In this section, you will find the regulations and guidelines that MEDELIA modules adhere to.
Regulations and Guidelines
The EU Regulation 2017/745 of April 5, 2017, regarding medical devices, establishes the requirements for post-market surveillance (PMS) and post-market clinical follow-up (PMCF) to ensure the continued safety and performance of medical devices. It includes detailed provisions on PMS and PMCF plans.
• Articles 83-86: Specify manufacturers’ obligations concerning PMS.
• Annex XIV, Part B: Details the requirements for PMCF.
Similar to the MDR, the IVDR requires manufacturers of in vitro diagnostic devices to implement post-market surveillance (PMS) and post-market clinical follow-up (PMCF) plans.
• Articles 78-81: Specify manufacturers’ obligations concerning PMS.
• Annex XIII, Part B: Details the requirements for PMCF.
Smart software for your business!
Contact us today - we'll guide you through each implementation phase with full support. Learn more by downloading our brochure or request a demo presentation!



